
What is the Connection Between Atopic Dermatitis and Asthma?
- July 31, 2025
- 1 Like
- 738 Views
- 0 Comments
Abstract
Purpose: This article aims to comprehensively explore the intricate connection between atopic dermatitis (AD) and asthma, two chronic inflammatory conditions that frequently co-exist and represent significant global health burdens. It seeks to elucidate the shared genetic, immunological, and environmental factors that underpin their strong epidemiological and mechanistic link, particularly within the well-established framework of the “atopic march.” By synthesizing the most current research from diverse fields including immunology, genetics, dermatology, pulmonology, and microbiology, this paper provides a detailed and nuanced understanding of the common pathophysiological mechanisms that drive both diseases and discusses the profound clinical implications for integrated management strategies, early intervention, and future therapeutic innovations.
Findings: A robust and increasingly understood epidemiological and mechanistic link exists between atopic dermatitis and asthma, often manifesting as a sequential progression known as the atopic march. This complex progression is driven by a dynamic interplay of systemic and tissue-specific factors. Key findings consistently indicate that genetic predispositions, most notably loss-of-function mutations in the filaggrin (FLG) gene, critically compromise both skin barrier and airway barrier functions. This impaired barrier integrity acts as a primary gateway, facilitating enhanced allergen penetration and subsequent immune sensitization. Furthermore, profound immune dysregulation, predominantly characterized by a dominant T helper 2 (Th2) immune response, involves the overexpression of key pro-inflammatory cytokines such as Interleukin-4 (IL-4), Interleukin-5 (IL-5), and Interleukin-13 (IL-13). These cytokines collectively drive chronic inflammation and tissue remodeling in both the skin and airways. Beyond genetics and core immunology, significant alterations in the microbiome (encompassing both skin and gut dysbiosis, with a particular emphasis on the pathogenic role of Staphylococcus aureus in AD) contribute significantly to immune priming, systemic sensitization, and disease exacerbation. Finally, a wide array of environmental triggers, including common allergens (e.g., dust mites, pollen), various irritants, and pervasive air pollutants, further interact synergistically with these compromised barriers and dysregulated immune systems. The cumulative and often synergistic effect of these multifaceted factors dramatically increases the risk for atopic comorbidities, leading to a substantial and often debilitating burden on patients’ physical health, psychological well-being, and overall quality of life.
Research Limitations/Implications: While extensive and rapidly advancing research robustly supports the concept of the atopic march and the existence of shared underlying mechanisms, there remains a critical need for more sophisticated, long-term longitudinal studies conducted across diverse global populations. Such studies are essential to fully delineate the precise temporal relationships between the onset of various atopic conditions, identify reliable predictive biomarkers for disease progression, and understand the impact of varying genetic and environmental contexts. Further research is also urgently required to unravel the complex interplay between specific genetic susceptibilities, nuanced early-life environmental exposures, and the dynamic evolution of microbial dysbiosis in different geographical and cultural settings. This deeper understanding is particularly crucial for the development of truly personalized prevention and targeted treatment strategies. The profound implications of these findings underscore the absolute necessity of early identification of at-risk individuals, the implementation of integrated and multidisciplinary management approaches, and the accelerated development of novel, targeted therapies that can effectively address shared underlying pathophysiological pathways to prevent or significantly mitigate the progression and severity of atopic diseases.
Practical Implications: Clinicians across various specialties, including pediatricians, dermatologists, allergists, and pulmonologists, must adopt an integrated, holistic, and collaborative approach to diagnosing and managing AD and asthma, explicitly recognizing their profound interconnectedness. Early and aggressive management of atopic dermatitis, particularly focusing on meticulous skin barrier repair and comprehensive inflammation control, holds significant potential to reduce the risk of subsequent asthma development. Comprehensive patient education on effective allergen avoidance strategies, environmental trigger mitigation, and the critical importance of strict adherence to comprehensive, long-term treatment plans is paramount. Furthermore, healthcare systems should be restructured to actively facilitate multidisciplinary care teams, enabling seamless collaboration among specialists to optimize patient outcomes, reduce disease burden, and significantly improve the overall quality of life for affected individuals and their families.
Social Implications: A deeper and more widespread understanding of the intricate AD-asthma connection can fundamentally inform and reshape public health initiatives aimed at early intervention, primary prevention, and improved management of atopic diseases on a population level. By systematically identifying high-risk individuals and implementing targeted, evidence-based strategies from infancy, societies can collectively reduce the escalating overall burden of chronic allergic conditions. This, in turn, translates into improved quality of life for affected individuals and their families, reduced healthcare system strain, and enhanced public health outcomes. This integrated knowledge also fosters a more holistic and comprehensive view of allergic diseases, actively encouraging more collaborative research endeavors, innovative clinical care models, and ultimately, a more effective global response to the rising tide of atopic conditions.
Originality/Value: This article synthesizes the current cutting-edge scientific understanding of the AD-asthma connection, drawing comprehensively from the latest advancements in immunology, genetics, molecular biology, dermatology, and pulmonology. It provides a comprehensive and accessible overview of the shared pathophysiological mechanisms and meticulously translates complex scientific principles into actionable insights for both clinicians and researchers. Its emphasis on integrated care, the exploration of emerging targeted therapies, and the identification of crucial future research directions makes it a valuable and timely resource for advancing the understanding, prevention, and management of these prevalent, debilitating, and interconnected atopic conditions.
Keywords: Atopic dermatitis, asthma, atopic march, genetic predisposition, filaggrin, Th2 immunity, immune dysregulation, skin barrier, airway barrier, microbiome, dysbiosis, Staphylococcus aureus, environmental triggers, inflammation, biologics, integrated care, IgE, eosinophils, TSLP, IL-4, IL-5, IL-13, personalized medicine, psychological impact.
1. Introduction: The Intertwined Nature of Atopic Dermatitis and Asthma
Atopic dermatitis (AD), commonly known as eczema, and asthma are two of the most prevalent chronic inflammatory diseases globally, significantly impacting the quality of life for millions of individuals across all age groups and socioeconomic strata. While AD primarily affects the skin, characterized by intense itching, persistent redness, and chronic inflammation that can lead to skin thickening and infection, and asthma targets the airways, leading to recurrent episodes of wheezing, shortness of breath, chest tightness, and coughing, these conditions are far from isolated entities. Epidemiological studies consistently reveal a strong, well-established, and increasingly understood link between the two, with individuals affected by AD having a substantially higher risk of developing asthma later in life, and vice versa. This sequential progression of allergic manifestations, often beginning with AD in infancy and frequently followed by food allergies, allergic rhinitis (hay fever), and then asthma in childhood or adolescence, is famously termed the “atopic march” (The Atopic March: Progression from Atopic Dermatitis to Allergic Rhinitis and Asthma, n.d.; Mechanisms by Which Atopic Dermatitis Predisposes to Food Allergy and the Atopic March, n.d.). This concept underscores a shared underlying predisposition to allergic diseases, manifesting differently across various organ systems over time.
The concept of the atopic march highlights that these seemingly distinct diseases are, in fact, different clinical expressions of a shared underlying predisposition to atopy – a genetic tendency to develop allergic hypersensitivity reactions upon exposure to common environmental substances. This predisposition involves a complex and dynamic interplay of inherited genetic susceptibility, profound immune system dysregulation (particularly a skew towards allergic or Type 2 immunity), compromised barrier functions in various organs (most notably the skin and respiratory tract), and a wide array of environmental exposures that can trigger or exacerbate symptoms. The cumulative burden imposed by AD and asthma is immense, extending far beyond the physical symptoms. It profoundly affects patients’ psychological well-being, disrupts sleep quality for both children and adults, impairs academic performance in school-aged children, reduces work productivity for adults, and significantly limits social interactions and participation in daily activities. The chronic, relapsing nature of these conditions often necessitates long-term management, frequent healthcare visits, adherence to complex treatment regimens, and significant financial expenditure on medications and specialist care, placing a substantial and growing strain on healthcare systems worldwide.
Understanding the deep and multifaceted connection between AD and asthma is crucial for several compelling reasons. Firstly, it moves beyond a purely symptomatic or organ-specific approach to treatment, allowing for a more holistic, proactive, and preventative management strategy. If clinicians and researchers can precisely identify the common pathways, genetic vulnerabilities, and environmental risk factors that drive both diseases, we might be able to intervene much earlier in the atopic march, potentially preventing or significantly mitigating the severity of subsequent atopic conditions before they become entrenched. Secondly, this interconnected understanding fundamentally informs the development of integrated care models, fostering essential collaboration and seamless communication between specialists such as dermatologists, allergists, pulmonologists, and other healthcare professionals. This multidisciplinary approach is vital for providing comprehensive, patient-centered care that addresses all facets of the patient’s atopic burden. Finally, unraveling these shared pathophysiological mechanisms opens exciting new avenues for the development of targeted therapeutic interventions that could simultaneously address multiple atopic manifestations, offering more effective and efficient treatment options for patients suffering from poly-atopic conditions.
This article aims to provide a comprehensive exploration of the intricate connection between atopic dermatitis and asthma. We will delve deeply into the concept of the atopic march, meticulously dissect the shared pathophysiological mechanisms at play (including genetic predispositions, immune dysregulation, barrier dysfunction, and the role of the microbiome and environmental triggers), discuss the critical clinical implications for diagnosis and integrated management, and highlight key areas for future research and therapeutic development. By shedding light on this intricate and dynamic relationship, we hope to contribute to a more integrated understanding and ultimately, more effective strategies for combating the growing global burden of atopic diseases, improving patient outcomes and quality of life.
2. The Atopic March: A Developmental Progression
The “atopic march” is a widely recognized and extensively studied clinical phenomenon describing the natural history and typical, albeit not universal, progression of allergic diseases in genetically predisposed individuals. It posits that atopic conditions often appear in a characteristic sequence over time, beginning early in life and evolving into different, yet interconnected, manifestations as a child ages. This concept provides a valuable framework for understanding the cumulative burden of allergic disease.
Typically, the atopic march is observed to begin with atopic dermatitis (AD) in infancy, often manifesting within the first few months of life, sometimes even as early as 2-3 months. This early-onset AD is characterized by intensely dry, persistently itchy, and inflamed skin, commonly appearing on the face (cheeks, forehead), scalp, and extensor surfaces (outer elbows and knees) in infants, evolving to flexural areas (creases of elbows and knees) in older children. The severity, persistence, and extent of this initial AD are often strong and reliable predictors of subsequent allergic diseases. For instance, infants with early-onset, severe, and widespread AD, particularly those with high levels of allergen-specific IgE antibodies, have a significantly higher risk of developing other atopic conditions (such as food allergies, allergic rhinitis, and asthma) compared to those with mild, transient, or later-onset eczema (The Atopic March: Progression from Atopic Dermatitis to Allergic Rhinitis and Asthma, n.d.; Mechanisms by Which Atopic Dermatitis Predisposes to Food Allergy and the Atopic March, n.d.).
Following AD, many children may develop food allergies, typically emerging in the first few years of life, often between 6 months and 2 years of age. Common food allergens include milk, egg, peanut, and tree nuts. This is then often followed by the development of allergic rhinitis (hay fever), characterized by symptoms such as persistent nasal congestion, sneezing, runny nose, and itchy eyes, usually appearing in preschool or early school-age years (around 3-5 years old). Finally, asthma often emerges later in childhood, commonly around school age (5-8 years old), though it can develop at any point during childhood or even adulthood. The progression is not absolute or universal; not every child with AD will develop all subsequent conditions, nor will they necessarily follow this exact sequence or timeline. Some children may skip stages, and some may have conditions resolve. However, the strong epidemiological association and the typical temporal pattern underscore a fundamental underlying connection and a shared predisposition to allergic inflammation across different organ systems.
The central hypothesis behind the atopic march suggests that the initial breach or dysfunction of the skin barrier in AD plays a pivotal and initiating role in triggering the systemic allergic sensitization process. A compromised skin barrier, often due to genetic defects (like FLG mutations) or environmental damage, allows environmental allergens (e.g., house dust mites, pet dander, pollens, food proteins, bacterial products like Staphylococcus aureus superantigens) to penetrate the skin more easily than in healthy individuals. This epicutaneous exposure to allergens, particularly in the context of a genetically predisposed and immature immune system, triggers a robust T helper 2 (Th2) immune response in the skin. This localized Th2-driven inflammation leads to the production of pro-inflammatory cytokines (IL-4, IL-13, TSLP) and, crucially, the generation of allergen-specific immunoglobulin E (IgE) antibodies. These IgE antibodies then circulate throughout the body, “priming” mast cells and other immune cells in distant mucosal surfaces. This systemic sensitization, initiated through the skin, makes the immune system hyper-responsive and primes it for allergic reactions in other mucosal surfaces, such as the gastrointestinal tract (leading to food allergies) and, most significantly for this discussion, the respiratory tract (leading to allergic rhinitis and asthma) upon subsequent exposure to the same or cross-reactive allergens via inhalation or ingestion (Mechanisms by Which Atopic Dermatitis Predisposes to Food Allergy and the Atopic March, n.d.).
While the concept of the atopic march is well-supported by numerous cross-sectional and longitudinal studies, the exact cellular and molecular mechanisms driving this complex progression are still areas of intense research. They involve a dynamic and intricate interplay between inherited genetic susceptibility, various environmental factors (including microbial exposures), the integrity and function of different epithelial barriers (skin, gut, lung), and the intricate balance and maturation of the immune system, particularly the delicate Th1/Th2 paradigm. Understanding these shared pathophysiological mechanisms is not merely an academic pursuit; it is absolutely key to developing effective, integrated prevention and treatment strategies that can potentially interrupt or modify the course of the atopic march, thereby reducing the overall burden of allergic diseases.
3. Shared Pathophysiological Mechanisms
The strong and consistent epidemiological link between atopic dermatitis and asthma is not merely a coincidental observation; it is deeply rooted in shared underlying pathophysiological mechanisms that create a common “atopic diathesis.” This diathesis represents a systemic predisposition to allergic inflammation that can manifest in different organ systems. Understanding these intricate, interwoven factors is crucial for targeted interventions.
3.1. Genetic Predisposition: The Blueprint for Atopy
Genetics play a profoundly significant role in determining an individual’s susceptibility to atopic diseases. A clear family history of AD, asthma, allergic rhinitis, or food allergies significantly increases a child’s risk of developing these conditions, indicating a strong inherited component. While atopy is a complex polygenic disorder, involving the interplay of multiple genes, one of the most extensively studied and impactful genetic factors identified to date is loss-of-function mutations in the filaggrin (FLG) gene (Genetics and Epigenetics of Atopic Dermatitis: An Updated Systematic Review, n.d.; Genetic factors predisposing to atopic dermatitis: selected genes related to the skin barrier and immune response, n.d.).
The FLG gene encodes for filaggrin, a crucial protein essential for the formation and integrity of the epidermal barrier, particularly within the outermost layer of the skin, the stratum corneum. Filaggrin plays multiple vital roles: it helps aggregate keratin filaments into dense bundles, which are critical for the structural integrity of skin cells; it contributes to the formation of the cornified envelope, a protective outer layer of keratinocytes; and it is a precursor to natural moisturizing factors (NMFs), such as urocanic acid and pyrrolidone carboxylic acid, which are vital for maintaining skin hydration and an acidic pH. Loss-of-function mutations in FLG lead to a deficiency or absence of functional filaggrin protein. This results in a fundamentally compromised skin barrier, characterized by increased transepidermal water loss (TEWL), chronic dryness, and heightened susceptibility to allergen penetration, irritant exposure, and microbial colonization (Pathogenesis and management of atopic dermatitis: insights into epidermal barrier dysfunction and immune mechanisms, n.d.; Genetic factors predisposing to atopic dermatitis: selected genes related to the skin barrier and immune response, n.d.). Critically, these FLG mutations are not only strongly associated with severe AD but are also a significant independent risk factor for the development of asthma, especially asthma that co-occurs with eczema (Skin Exposure and Asthma: Is There a Connection?, n.d.). This highlights a direct and powerful genetic link between primary skin barrier defects and the subsequent development of airway disease, supporting the atopic march hypothesis.
Beyond FLG, extensive genome-wide association studies (GWAS) have identified numerous other genetic loci that are shared risk factors for both AD and asthma. These genes are often involved in critical biological processes such as immune regulation, inflammatory pathways, epithelial cell differentiation and function, and innate immune signaling. Examples include genes related to the Th2 immune response (e.g., IL4, IL13, IL4R, STAT6), epithelial alarmins (e.g., TSLP, IL33, IL25), and components of the innate immune system (Genetic factors predisposing to atopic dermatitis: selected genes related to the skin barrier and immune response, n.d.; Mechanisms by Which Atopic Dermatitis Predisposes to Food Allergy and the Atopic March, n.d.). This complex and overlapping genetic landscape underscores a common underlying predisposition and vulnerability to allergic inflammation that can manifest across various organ systems.
3.2. Immune Dysregulation: The Dominance of Th2 and Beyond
A defining hallmark of both AD and asthma is a predominant T helper 2 (Th2) immune response. In healthy individuals, the immune system maintains a finely tuned balance between different T helper cell subsets (e.g., Th1, Th2, Th17, Treg), each mediating distinct types of immune responses. However, in atopic individuals, there is a distinct and persistent skew towards Th2 cell activation, which drives the allergic inflammatory cascade.
Th2 cells produce a specific and potent set of cytokines that are central to allergic inflammation in both the skin and airways:
- Interleukin-4 (IL-4): This cytokine is crucial for initiating and sustaining the Th2 response. It plays a pivotal role in promoting IgE class switching in B cells, leading to the production of high levels of allergen-specific IgE antibodies, a hallmark of atopy. IL-4 also contributes to barrier dysfunction in both the skin and lungs by disrupting tight junctions between epithelial cells, making them more permeable (Type 2 immunity in the skin and lungs, n.d.; The Roles of Th2-Type Cytokines in the Pathogenesis of Atopic Dermatitis, n.d.).
- Interleukin-5 (IL-5): This cytokine is primarily responsible for the growth, differentiation, recruitment, and prolonged survival of eosinophils. Eosinophils are key inflammatory cells that contribute significantly to tissue damage and inflammation in both AD skin lesions (where they are abundant) and asthmatic airways (where their presence is a characteristic feature of allergic asthma) (Type 2 immunity in the skin and lungs, n.d.).
- Interleukin-13 (IL-13): Sharing many functional similarities with IL-4, IL-13 is a critical mediator of allergic inflammation. In the airways, it promotes excessive mucus hypersecretion by goblet cells, contributes to airway hyperresponsiveness (the tendency of airways to constrict easily), and plays a role in airway remodeling. In the skin, IL-13 further contributes to barrier defects and inflammation (Type 2 immunity in the skin and lungs, n.d.).
This persistent Th2-dominant environment leads to elevated levels of total IgE and allergen-specific IgE antibodies in the serum, significant eosinophilia in blood and affected tissues, and chronic, relapsing inflammation. While Th2 responses are undeniably central, more recent research also highlights the complex involvement of other immune cells and pathways. These include Th17 cells (which produce IL-17 and contribute to neutrophilic inflammation and barrier disruption), Innate Lymphoid Cells Type 2 (ILC2s) (which are rapid producers of Th2 cytokines in response to epithelial alarmins, even before adaptive T cell responses are fully mounted), and a potential dysfunction in regulatory T cells (Tregs) (which normally suppress immune responses), all contributing to the intricate immune dysregulation observed in both conditions (Type 2 immunity in the skin and lungs, n.d.). The persistent activation of these interconnected pathways creates a systemic inflammatory milieu that affects multiple organ systems, explaining the multi-organ manifestations of atopy.
3.3. Barrier Dysfunction: The Leaky Gates of Skin and Airway Integrity
A compromised epithelial barrier is a profoundly critical shared mechanism in the pathogenesis of both AD and asthma, serving as a primary gateway for allergen entry, microbial colonization, and subsequent immune sensitization.
- Skin Barrier Dysfunction in AD: As previously highlighted, genetic mutations in FLG are a major predisposing factor for epidermal barrier dysfunction in AD. However, even in the absence of FLG mutations, the skin barrier can be compromised by other intrinsic factors, such as reduced levels of key epidermal lipids (e.g., ceramides, cholesterol, free fatty acids) that form the crucial lipid lamellae, altered lipid composition, and increased activity of proteases that degrade barrier proteins. This results in a “leaky” skin barrier, characterized by increased transepidermal water loss (TEWL) and reduced defense against external insults. This compromised barrier allows environmental allergens (e.g., house dust mites, pollens, pet dander), irritants (e.g., detergents, chemicals), and microbes (e.g., Staphylococcus aureus) to more easily penetrate the epidermis. Once inside, these foreign substances activate underlying immune cells (e.g., Langerhans cells, dendritic cells, mast cells), initiating or exacerbating the Th2 immune response. This epicutaneous sensitization, occurring through the skin, is widely hypothesized to be the initial and pivotal step in the atopic march, priming the immune system systemically for allergic reactions in other organs (Pathogenesis and management of atopic dermatitis: insights into epidermal barrier dysfunction and immune mechanisms, n.d.; Skin Exposure and Asthma: Is There a Connection?, n.d.).
- Airway Barrier Dysfunction in Asthma: Strikingly similar to the skin, the airway epithelium in asthmatic individuals often exhibits compromised barrier integrity. Factors such as chronic inflammation, recurrent viral infections (e.g., rhinovirus, respiratory syncytial virus), and prolonged exposure to air pollutants can directly damage the tight junctions and adherens junctions that normally seal the gaps between epithelial cells. This “leaky airway barrier” allows inhaled allergens, viruses, and irritants to more easily penetrate the airway lining, gaining direct access to underlying immune cells. This penetration triggers robust local immune responses, chronic inflammation, mucus hypersecretion, and airway hyperresponsiveness, all characteristic features of asthma (Type 2 immunity in the skin and lungs, n.d.). The concept of a “leaky barrier” is thus a unifying theme, central to the pathology of both skin and lung in atopic diseases, facilitating systemic sensitization and organ-specific inflammation.
3.4. The Microbiome Axis: Skin and Gut Dysbiosis as Immune Modulators
The human microbiome, the vast and dynamic community of microorganisms (bacteria, fungi, viruses) inhabiting our bodies, plays a profoundly crucial role in immune system development, maturation, and maintaining host homeostasis. Dysbiosis, defined as an imbalance or alteration in the composition and function of these microbial communities, in both the skin and gut, is increasingly recognized as a significant contributing factor to the development and exacerbation of atopic diseases. This highlights the importance of the “hygiene hypothesis” and the role of microbial exposures in early life immune programming.
- Skin Microbiome (Dysbiosis in AD): The skin of AD patients consistently exhibits reduced microbial diversity and a significant overabundance of Staphylococcus aureus (S. aureus) compared to healthy skin, even in unaffected areas (Microbiome in the Gut-Skin Axis in Atopic Dermatitis, n.d.; Interactions Between Atopic Dermatitis and Staphylococcus aureus Infection: Clinical Implications, n.d.). S. aureus colonization is strongly and positively correlated with AD severity and the frequency of disease flares. This opportunistic bacterium produces a variety of virulence factors, including exotoxins (e.g., alpha-toxin, delta-toxin), proteases, and potent superantigens (e.g., staphylococcal enterotoxins). These factors can directly damage the already compromised skin barrier, activate keratinocytes and underlying immune cells (e.g., T cells, mast cells), promote a strong Th2/Th17 inflammatory response, and exacerbate the cardinal symptoms of itching and inflammation. Furthermore, S. aureus superantigens can act as powerful allergens, contributing directly to IgE sensitization and potentially linking skin colonization to systemic allergic responses, including those relevant to asthma, by promoting systemic Th2 skewing (Staphylococcus aureus and its IgE-inducing enterotoxins in asthma: current knowledge, n.d.).
- Gut Microbiome (The Gut-Skin-Lung Axis): The gut microbiome is a critical and powerful modulator of systemic immunity, influencing immune responses far beyond the gastrointestinal tract. Alterations in gut microbial composition, particularly during critical windows of immune development in early life (e.g., infancy), have been consistently linked to an increased risk of developing both AD and asthma. A less diverse gut microbiome, or specific microbial profiles (e.g., lower abundance of certain beneficial commensal bacteria like Bifidobacterium, Lactobacillus, and Faecalibacterium prausnitzii, or an overgrowth of certain pathogenic species), can profoundly influence immune maturation, potentially skewing the developing immune system towards a Th2-dominant, pro-allergic phenotype. The gut-skin-lung axis hypothesis proposes that metabolites produced by gut bacteria (e.g., short-chain fatty acids like butyrate, propionate, acetate), as well as microbial components and immune cells primed in the gut, can have systemic effects, influencing immune responses and barrier integrity in distant sites like the skin and lungs (Microbiome in the Gut-Skin Axis in Atopic Dermatitis, n.d.; Microbiome of the Skin and Gut in Atopic Dermatitis (AD): Understanding the Pathophysiology and Finding Novel Management Strategies, n.d.). Dysbiosis in the gut can thus contribute to systemic inflammation, promote allergic sensitization, and reinforce the intricate link between AD and asthma by modulating the overall immune tone.
3.5. Chronic Inflammation and Epithelial Alarmins: The Amplifiers
Both AD and asthma are fundamentally characterized by chronic, persistent inflammation driven by the sustained interplay of activated immune cells and a cascade of inflammatory mediators. A key initiating event in this inflammatory cycle is the release of “alarmin” cytokines by epithelial cells when they are damaged or stressed by allergens, irritants, or microbes. These alarmins act as danger signals, initiating and amplifying the Th2 immune response. Key alarmins that are elevated in both AD and asthma include:
- Thymic Stromal Lymphopoietin (TSLP): Released predominantly by epithelial cells in both the skin (keratinocytes) and airways (bronchial epithelial cells), TSLP is a potent and crucial inducer of Th2 responses. It directly activates innate lymphoid cells type 2 (ILC2s) and dendritic cells, which then efficiently prime naive T cells towards a Th2 phenotype. Increased TSLP expression is consistently observed in inflamed AD skin and asthmatic airways, playing a major role in driving systemic Th2 immunity and, consequently, the atopic march (Mechanisms by Which Atopic Dermatitis Predisposes to Food Allergy and the Atopic March, n.d.).
- Interleukin-25 (IL-25) and Interleukin-33 (IL-33): These are also powerful epithelial-derived alarmins that promote and sustain Th2 inflammation. They act on ILC2s, mast cells, and other immune cells to induce the rapid release of Th2 cytokines (IL-4, IL-5, IL-13), thereby amplifying the allergic response (Type 2 immunity in the skin and lungs, n.d.).
The continuous release of these alarmins, coupled with the persistent presence of Th2 cytokines, creates a self-perpetuating, vicious cycle of inflammation, ongoing barrier dysfunction, and pathological tissue remodeling in both the skin and airways. In the skin, this manifests as chronic itching, scratching, and subsequent skin thickening (lichenification), further damaging the already compromised barrier and increasing susceptibility to infection. In the airways, this leads to persistent mucus hypersecretion, smooth muscle constriction (bronchospasm), and structural changes in the airway walls (airway remodeling), all contributing to chronic asthma symptoms, reduced lung function, and frequent exacerbations.
3.6. Environmental Triggers and Exposures: The External Catalysts
Environmental factors play a crucial and often dynamic role in triggering and exacerbating both AD and asthma, frequently interacting synergistically with genetic predispositions and compromised barriers.
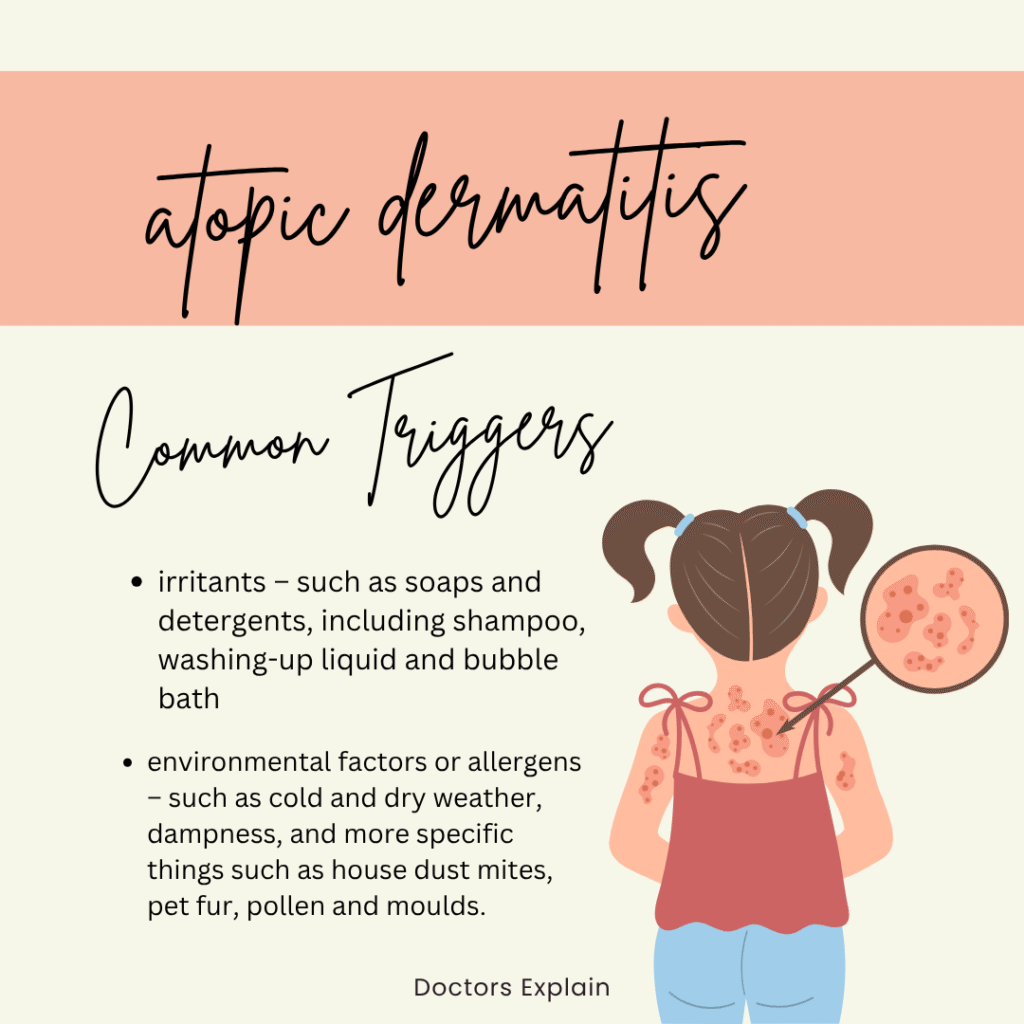
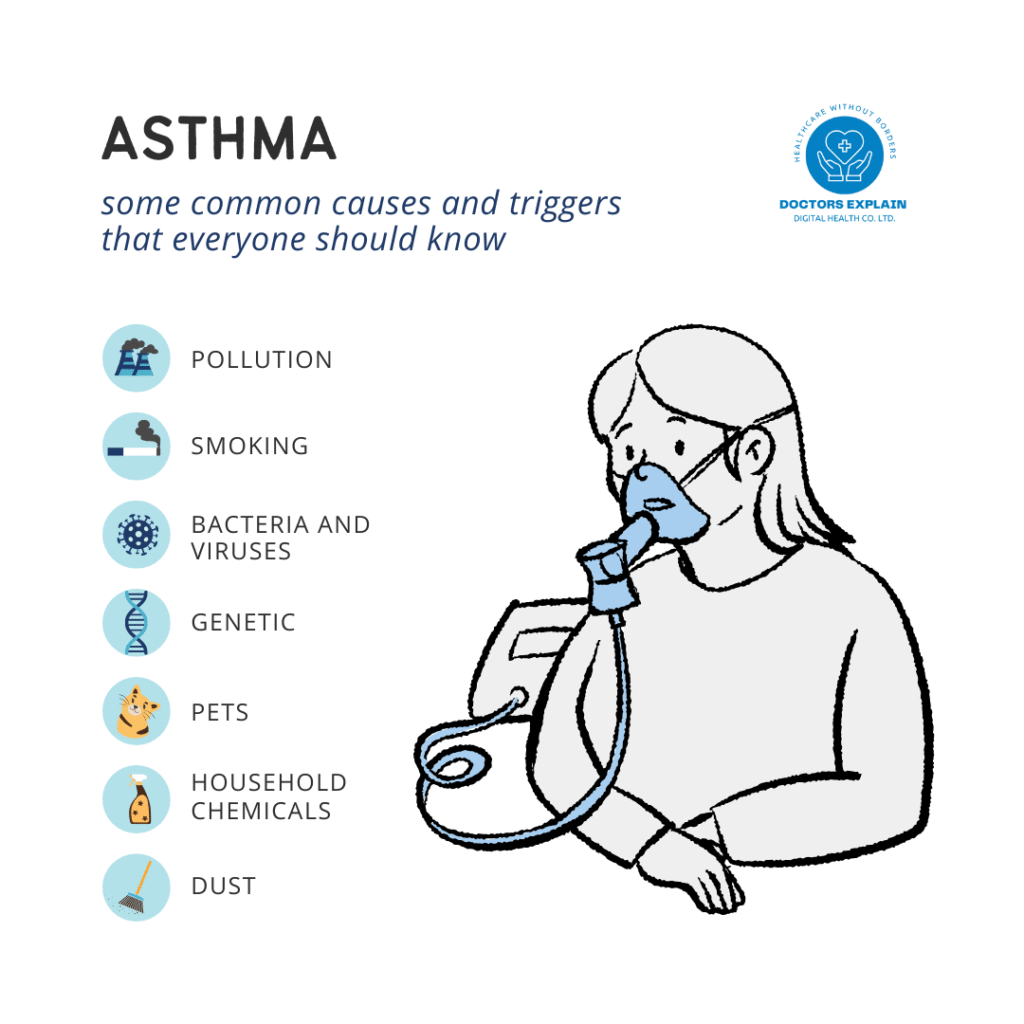
- Common Allergens: Exposure to ubiquitous environmental allergens is a major driver of symptoms in sensitized individuals. These include house dust mites (a primary trigger for both AD flares and asthma attacks), pollen (seasonal exacerbations of AD and allergic rhinitis/asthma), pet dander (from cats, dogs, etc.), and certain food proteins (which can trigger both AD flares and systemic allergic reactions, especially in children with a leaky skin barrier). The compromised skin barrier in AD facilitates sensitization to these allergens via the epicutaneous route, which can then trigger respiratory symptoms upon subsequent inhalation (Skin Exposure and Asthma: Is There a Connection?, n.d.).
- Irritants: Direct exposure to various irritants can exacerbate both conditions. For the skin, this includes harsh soaps, detergents, solvents, fragrances, and certain chemicals, all of which can directly damage the epidermal barrier and induce inflammation, worsening AD. Similarly, respiratory irritants such as strong fumes, cleaning products, tobacco smoke (first- and secondhand), and occupational chemicals can trigger asthma symptoms and airway inflammation.
- Air Pollution: There is a growing and robust body of evidence linking exposure to ambient air pollutants to both the development and exacerbation of AD and asthma (Air Pollution Linked to Worse Atopic Dermatitis, n.d.; Air Pollution and Atopic Dermatitis, from Molecular Mechanisms to Population-Level Evidence: A Review, n.d.). Pollutants such as particulate matter (PM2.5, PM10), nitrogen dioxide (NO2), ozone (O3), and sulfur dioxide (SO2) can induce oxidative stress, promote inflammation, and directly damage epithelial barriers in both the skin and lungs. This damage makes individuals more susceptible to allergen penetration and triggers inflammatory responses, thereby increasing the incidence and severity of both AD and asthma. Urbanization and industrialization contribute significantly to increased exposure to these pollutants.
- Climate Factors and Weather Changes: Climatic variables like temperature, humidity, and seasonal changes can also profoundly influence disease activity. For example, extreme temperatures (very hot or very cold) or very low humidity can worsen AD severity by increasing skin dryness and barrier dysfunction. Conversely, high humidity can promote dust mite growth. Seasonal pollen counts are major triggers for allergic rhinitis and asthma, and their impact can be exacerbated by climate change altering pollen seasons (Air Pollution Linked to Worse Atopic Dermatitis, n.d.).
- Infections: Viral infections (e.g., rhinovirus, RSV) are well-known triggers for asthma exacerbations, and bacterial infections (e.g., Staphylococcus aureus) are common in AD. These infections can further compromise barriers and amplify inflammatory responses, creating a vicious cycle.
The cumulative and often synergistic effect of these diverse environmental exposures, particularly in individuals with genetic susceptibility and underlying barrier defects, contributes significantly to the manifestation, persistence, and progression of atopic diseases across the lifespan.
4. Clinical Implications and Integrated Management
Recognizing the profound and intricate connection between atopic dermatitis and asthma has significant and transformative clinical implications. It necessitates a fundamental shift in the paradigm of care, moving away from treating isolated conditions in a fragmented manner towards adopting a truly integrated, holistic, and patient-centered management approach.
4.1. Early Identification and Risk Stratification: Intercepting the March
Given the well-established concept of the atopic march, the early identification of infants and young children at high risk for developing multiple atopic diseases is of paramount importance. This proactive approach allows for timely interventions that could potentially intercept or modify the disease trajectory. Factors that consistently indicate a higher risk for subsequent atopic comorbidities include:
- Early-onset, severe, and persistent AD: This is arguably the strongest predictor. If AD appears within the first few months of life, is widespread across the body, and proves difficult to control with standard treatments, the risk for developing asthma, food allergies, and allergic rhinitis significantly increases.
- Extensive AD lesions: The greater the body surface area affected by AD, the higher the likelihood of systemic sensitization and progression to other atopic conditions.
- Presence of FLG gene mutations: While not routinely screened in all clinical settings, a strong family history of severe AD, ichthyosis vulgaris (a common skin condition associated with FLG mutations), or other atopic diseases can be indicative. Genetic testing for FLG mutations may be considered in research settings or for specific clinical indications.
- High levels of total IgE or allergen-specific IgE: Elevated serum levels of total immunoglobulin E (IgE), or specific IgE antibodies to common environmental (e.g., house dust mites, cat/dog dander, pollens) or food allergens (e.g., milk, egg, peanut), particularly in early childhood, are strong indicators of systemic allergic sensitization and increased atopic risk.
- Family history of asthma or other severe atopic diseases: A first-degree relative (parent or sibling) with severe asthma, AD, or other allergic conditions substantially increases a child’s genetic predisposition.
Pediatricians and primary care providers play an absolutely crucial role as the first point of contact in identifying these risk factors during routine well-child visits. They should be vigilant for early signs of AD and proactively inquire about family history of atopy. Routine screening for respiratory symptoms (e.g., recurrent cough, wheezing, shortness of breath) in children with AD is essential, even if they haven’t yet received a formal asthma diagnosis, enabling earlier intervention.
4.2. Integrated Therapeutic Approaches: Targeting Shared Pathways
Managing AD and asthma as interconnected conditions requires a truly multidisciplinary and integrated approach, fostering seamless collaboration among specialists such as dermatologists, allergists/immunologists, pulmonologists, and often involving dietitians, psychologists, and specialized nurses. The goal is to optimize overall patient outcomes and improve quality of life by addressing shared underlying mechanisms.
- Aggressive Skin Barrier Repair and Inflammation Control in AD: Early and consistent management of AD, with a primary focus on restoring and maintaining skin barrier function, is a cornerstone strategy. This involves the liberal and regular use of emollients and moisturizers (e.g., those containing ceramides, filaggrin-like components, or natural moisturizing factors) to hydrate the skin, reduce transepidermal water loss, and physically reinforce the barrier. Effective topical anti-inflammatory treatments (e.g., topical corticosteroids of appropriate potency, topical calcineurin inhibitors, topical PDE4 inhibitors) are crucial to control inflammation, reduce intense itching, and thereby break the “itch-scratch cycle” that further damages the barrier. Some compelling research suggests that early and consistent skin barrier intervention in infants with AD might potentially reduce the risk of subsequent food allergy and asthma development by preventing epicutaneous sensitization, though more definitive large-scale, long-term clinical trials are still ongoing to fully confirm this “dual-allergen exposure hypothesis.”
- Comprehensive Allergen Avoidance and Environmental Control: Identifying and minimizing exposure to relevant allergens (e.g., house dust mites, pet dander, pollens, molds) and environmental irritants (e.g., tobacco smoke, strong fumes, air pollutants) in both the home and, where applicable, the workplace environments is critical for both AD and asthma. This can involve practical measures like using allergen-proof bedding covers, regular vacuuming with HEPA filters, maintaining optimal indoor humidity, avoiding indoor pets if sensitized, and ensuring good indoor air quality through ventilation or air purifiers. For food allergies, strict dietary avoidance of confirmed allergens is necessary.
- Targeted Anti-Inflammatory Treatments: Both conditions fundamentally benefit from anti-inflammatory therapies. For AD, this includes the topical medications mentioned above, and in more severe cases, systemic immunosuppressants or targeted biologics. For asthma, inhaled corticosteroids (ICS) remain the cornerstone of long-term control therapy, reducing airway inflammation and preventing exacerbations. The shared inflammatory pathways mean that some systemic treatments might benefit both conditions, offering a more streamlined approach to poly-atopic patients.
- Addressing the Microbiome: A Growing Frontier: While still an area of active and evolving research, strategies targeting the microbiome, such as the use of specific probiotics (especially in early life, though strain-specific efficacy is crucial) or prebiotics, are being explored for their potential to modulate immune responses, promote immune tolerance, and influence the natural course of atopic diseases. For AD, managing Staphylococcus aureus colonization on the skin with antiseptic washes (e.g., dilute bleach baths, chlorhexidine) or targeted topical/systemic antibiotics (when indicated for infection) can also be important to reduce inflammation and flares.
- Patient Education and Empowered Self-Management: Empowering patients and their caregivers with comprehensive, clear, and actionable education about both conditions, their specific triggers, the rationale behind treatments, and effective self-management strategies is absolutely vital for long-term success. This includes developing personalized written action plans for managing AD flares and asthma exacerbations, ensuring proper inhaler technique, teaching skin care routines, and promoting strict adherence to prescribed treatment regimens. Understanding their conditions empowers patients to take an active role in their health.
4.3. Emerging Targeted Therapies: The Dawn of Biologics
Advances in understanding the precise molecular mechanisms and shared immune pathways driving atopic inflammation have led to the revolutionary development of highly targeted therapies, particularly biologics. These innovative medications offer new hope for patients with severe, uncontrolled AD and asthma who do not respond adequately to conventional treatments.
- Dupilumab (Dupixent): This groundbreaking biologic is a monoclonal antibody that targets the Interleukin-4 receptor alpha (IL-4Rα) subunit. By blocking this common receptor, dupilumab effectively inhibits signaling from both IL-4 and IL-13, two central Th2 cytokines. It is approved for the treatment of moderate-to-severe AD in adults and children, and for severe asthma with a Th2 phenotype. Its remarkable efficacy in simultaneously treating both conditions powerfully underscores the central role of IL-4 and IL-13 in driving the chronic inflammation characteristic of atopic diseases (Biologics – Northeast Allergy, Asthma, and Immunology, n.d.; Biologics for Asthma & Eczema, n.d.).
- Omalizumab (Xolair): This anti-IgE monoclonal antibody is approved for severe allergic asthma and chronic spontaneous urticaria (hives). By binding to free IgE in the bloodstream, omalizumab prevents IgE from binding to its receptors on mast cells and basophils, thereby reducing the release of inflammatory mediators during allergic reactions and dampening the allergic cascade.
- Mepolizumab (Nucala), Reslizumab (Cinqair), Benralizumab (Fasenra): These biologics specifically target Interleukin-5 (IL-5) or the IL-5 receptor. By neutralizing IL-5 or depleting eosinophils (the cells that IL-5 primarily acts upon), these therapies significantly reduce eosinophil levels and activity. They are approved for severe eosinophilic asthma and are being explored for other eosinophil-driven conditions, including some forms of AD.
These targeted biologic therapies represent a significant paradigm shift towards personalized medicine in atopy. By precisely addressing the specific immune drivers common to both AD and asthma, they offer the potential for more effective, safer, and simultaneously acting treatments, leading to improved disease control and quality of life for poly-atopic patients.
5. Psychological and Social Impact
Beyond the visible skin lesions and respiratory symptoms, chronic atopic conditions like atopic dermatitis and asthma exert a profound and often debilitating psychological and social burden on affected individuals and their families. This frequently overlooked aspect of the disease significantly impacts overall quality of life and can complicate adherence to treatment and overall disease management.
- Psychological Distress and Comorbidities: Both AD and asthma are strongly associated with increased rates of anxiety and depression across all age groups and disease severities (The Psychology of Atopic Dermatitis, n.d.; Understanding the psychological toll of asthma or allergies, n.d.). The relentless and often uncontrollable itch of AD, particularly at night, leads to chronic sleep disturbance and deprivation, which in turn exacerbates irritability, fatigue, poor concentration, and significantly contributes to mood disorders. The unpredictable nature of AD flares and sudden asthma attacks can create profound anxiety, constant fear (e.g., fear of an attack in public, fear of suffocation), and a pervasive sense of loss of control over one’s body and life. Patients may also develop alexithymia, a psychological construct characterized by a difficulty in identifying, expressing, and understanding one’s own emotions, which can further hinder their ability to communicate their distress to healthcare providers or family members (The Psychology of Atopic Dermatitis, n.d.). Children with AD are at a higher risk for experiencing bullying, developing poor self-image due to visible skin lesions, and exhibiting social withdrawal, impacting their academic performance and social development. For asthma patients, the constant worry of an attack, especially in social or public settings, can lead to significant social anxiety, avoidance of physical activities, and a restricted lifestyle.
- Profound Impact on Quality of Life (QoL): The chronic, relapsing, and often unpredictable nature of these diseases significantly impairs the overall quality of life for patients and their families. Daily activities, participation in schooling, work productivity, social interactions, and engagement in sports or hobbies can be severely restricted. Children may miss school days, and adults may miss work, leading to academic and professional setbacks. Parents of children with severe AD and asthma often experience considerable chronic stress, severe sleep deprivation (due to caring for itching or wheezing children at night), and significant financial strain due to the high costs of medications, specialist visits, and time off work. This can lead to parental depression, marital strain, and overall negative impacts on family dynamics and household well-being (The Psychology of Atopic Dermatitis, n.d.).
- Stigma and Social Isolation: The visible skin lesions, redness, and excoriations associated with AD can unfortunately lead to significant social stigma, misunderstanding, and isolation. Others may mistakenly believe the condition is contagious, a result of poor hygiene, or simply “cosmetic,” leading to judgmental stares, avoidance, and hurtful comments. Similarly, the audible symptoms of asthma (e.g., wheezing, coughing) or the need to use an inhaler in public can lead to feelings of embarrassment, self-consciousness, or being perceived as “different” or “sick.” This pervasive stigma can result in reduced self-esteem, body image issues, and a reluctance to engage in social activities, sports, or intimate relationships, further exacerbating feelings of loneliness and depression.
- Treatment Burden and Adherence Challenges: Managing chronic AD and asthma often involves complex and demanding daily routines. This can include applying multiple topical medications several times a day, taking oral medications, using various inhaled corticosteroids and bronchodilators, and meticulously implementing allergen avoidance strategies. This significant treatment burden can be overwhelming, particularly for children and their caregivers, leading to poor adherence to prescribed regimens, which in turn can result in suboptimal disease control and increased flares or exacerbations. The psychological distress associated with the disease can also negatively impact motivation for adherence.
Recognizing and proactively addressing these profound psychological and social impacts is an absolutely crucial component of holistic patient care. Healthcare providers should routinely screen for mental health comorbidities (e.g., using validated questionnaires for anxiety and depression), encourage open and empathetic conversations about the emotional toll of the disease, and facilitate timely referrals to mental health professionals (e.g., psychologists, counselors, social workers) when needed. Integrated care models should explicitly include psychosocial support services, such as cognitive-behavioral therapy (CBT), stress management techniques, support groups, and educational programs, to improve overall well-being, enhance coping mechanisms, and ultimately improve treatment adherence and long-term disease outcomes.
6. Research Gaps and Future Directions
Despite significant and rapid advancements in understanding the intricate connection between atopic dermatitis and asthma, several critical research gaps remain. Addressing these gaps will be absolutely crucial for developing more effective, personalized, and preventative strategies for these prevalent and often debilitating conditions. The journey towards a complete understanding and definitive cure is ongoing.
- Longitudinal Studies Across Diverse Populations and Life Stages: While the atopic march is well-described, there is a pressing need for more extensive, long-term longitudinal studies conducted across diverse global populations, including underrepresented ethnic groups and different socioeconomic strata. Such studies are essential to precisely delineate the temporal relationships between the onset of various atopic conditions, identify reliable predictive biomarkers for disease progression (e.g., genetic, epigenetic, immunological, microbial signatures), and understand if the march differs in its manifestation, severity, and progression across various ethnicities, geographical regions, and environmental contexts. Furthermore, research needs to extend beyond childhood to track the persistence and evolution of atopic diseases into adolescence and adulthood, and to understand how early-life interventions impact long-term outcomes.
- Precision Medicine and Personalized Interventions: The inherent heterogeneity of AD and asthma phenotypes (e.g., different endotypes based on specific molecular pathways) suggests that a “one-size-fits-all” approach to prevention and treatment is inherently insufficient. Future research should focus intensely on identifying specific endotypes that are shared between AD and asthma, allowing for more targeted therapeutic strategies. This will pave the way for true personalized medicine, where prevention and treatment strategies are tailored based on an individual’s unique genetic profile, immune signature, specific allergen sensitivities, and microbiome composition. This will likely involve advanced multi-omics approaches (genomics, transcriptomics, proteomics, metabolomics, microbiomics) to identify actionable biomarkers.
- Intricate Interplay of Genetics, Environment, and Microbiome: A deeper and more granular understanding of the complex, bidirectional interactions between genetic susceptibility (e.g., specific FLG mutations or other barrier gene variants), early-life environmental exposures (e.g., specific allergens, indoor/outdoor air pollutants, early microbial exposures, dietary patterns), and the dynamic development of both skin and gut dysbiosis is critically needed. This includes exploring the precise impact of specific microbial strains or their metabolites on immune programming, barrier function, and inflammation. For instance, how do early-life antibiotic use or mode of delivery impact the gut microbiome and subsequent atopic risk? How do specific components of air pollution interact with genetic predispositions to trigger disease?
- Early-Life Interventions for Primary Prevention: Research into early-life interventions, particularly those targeting skin barrier function (e.g., universal application of specific emollients or topical treatments from birth in high-risk infants) or microbiome modulation (e.g., specific probiotic strains, prebiotic supplementation, or even fecal microbiota transplantation in controlled settings), to prevent the initial onset or modify the progression of the atopic march requires robust, large-scale, and rigorously designed clinical trials. Understanding the optimal timing, duration, and specific agents for such interventions is paramount. This also includes exploring the role of early allergen introduction in food allergy prevention and its potential impact on the atopic march.
- Biomarker Discovery and Validation for Prediction and Response: Identifying reliable, non-invasive, and easily measurable biomarkers that can accurately predict disease severity, predict the likelihood of progression along the atopic march, and predict response to specific therapies for both AD and asthma is essential for guiding clinical decisions and developing truly targeted interventions. This includes genetic biomarkers, immunological markers (e.g., specific cytokine profiles, IgE subtypes), and microbial biomarkers. The development of robust diagnostic tools based on these biomarkers will revolutionize atopic disease management.
- Effectiveness of Psychosocial Interventions: While the psychological burden is recognized, more rigorous research is needed on the effectiveness of specific psychological and social interventions (e.g., tailored cognitive-behavioral therapy (CBT) for chronic itch or anxiety, mindfulness-based stress reduction, targeted support groups, comprehensive patient education programs) in improving the quality of life, mental health outcomes, and long-term treatment adherence for patients living with both AD and asthma. This also includes developing interventions for caregivers.
- Impact of Global Environmental Changes: Given the increasing prevalence of both conditions and the indisputable role of environmental triggers, further research is urgently needed to understand the long-term impact of global climate change, rapid urbanization, and increasing levels of air pollution on the incidence, severity, and geographical distribution of AD and asthma worldwide. This will inform public health policies and environmental regulations aimed at mitigating these risks.
Addressing these complex and multifaceted research gaps will require unprecedented collaborative, multidisciplinary efforts, leveraging advanced genomic, proteomic, metabolomic, and microbiomic technologies, alongside sophisticated epidemiological studies, large-scale birth cohorts, and robust clinical trial designs. The ultimate goal is to move towards a future where the atopic march can be effectively intercepted, and individuals with AD and asthma can achieve optimal health outcomes and a significantly improved quality of life.
7. Conclusion: Towards Integrated Care for Atopic Diseases
The intricate connection between atopic dermatitis and asthma is undeniable, rooted in a complex and dynamic tapestry of shared genetic predispositions, intricate immune dysregulation, compromised epithelial barrier functions across multiple organs, and the pervasive influence of the microbiome and a diverse array of environmental triggers. The concept of the “atopic march” serves as a powerful and illuminating framework, vividly illustrating how early-life skin barrier defects and subsequent epicutaneous allergen sensitization can profoundly prime the immune system for the later development of respiratory allergies, including allergic rhinitis and, crucially, asthma. Systemic factors such as loss-of-function mutations in the FLG gene and a persistent, overactive T helper 2 (Th2) immune response, coupled with local tissue-specific vulnerabilities and microbial imbalances (particularly Staphylococcus aureus dysbiosis in the skin), collectively create a fertile and permissive ground for the manifestation and progression of both conditions.
Recognizing this profound and multifaceted interconnectedness is not merely an academic exercise; it carries significant and transformative clinical implications that demand a fundamental shift in the paradigm of care. This mandates moving decisively away from siloed, organ-specific specialty care towards an integrated, holistic, and truly multidisciplinary approach that addresses the whole patient and the full spectrum of their atopic burden. Early identification of high-risk individuals, proactive and meticulous skin barrier repair strategies in infants with AD, comprehensive and sustained allergen avoidance measures, and the judicious and timely use of anti-inflammatory treatments are all crucial, interconnected steps in mitigating disease progression and improving long-term outcomes. The advent of highly targeted biologics, which precisely block shared inflammatory pathways (such as those mediated by IL-4 and IL-13), further underscores the common underlying biology of these conditions and offers incredibly promising avenues for simultaneous and more effective treatment of poly-atopic patients.
Beyond the visible physical symptoms and physiological dysfunctions, the substantial and often debilitating psychological and social burden of chronic AD and asthma necessitates a compassionate and holistic approach to care that explicitly includes routine screening for mental health comorbidities and the provision of robust psychosocial support. Future research must continue to unravel the intricate interplay of genetic predispositions, specific environmental exposures, and the dynamic role of the microbiome, paving the way for truly personalized prevention strategies and more effective, targeted therapies that can precisely intercept the atopic march at its earliest stages. This will lead to a future where the progression of atopic diseases can be significantly altered, and individuals affected by these pervasive conditions can achieve not only optimal physical health but also a significantly improved quality of life, free from the debilitating cycle of chronic inflammation and its associated distress. By embracing an integrated understanding and a collaborative management paradigm, we can move closer to a future where individuals with atopic dermatitis and asthma can achieve optimal health and a significantly improved quality of life.
References
Acas. (n.d.). Mental health adjustments – Reasonable adjustments at work. Retrieved July 26, 2025, from https://www.acas.org.uk/reasonable-adjustments/mental-health-adjustments
Acas. (n.d.). Mental health and the law – Supporting mental health at work. Retrieved July 26, 2025, from https://www.acas.org.uk/supporting-mental-health-workplace
Acas. (n.d.). Talking about mental health – Supporting mental health at work. Retrieved July 26, 2025, from https://www.acas.org.uk/supporting-mental-health-workplace/managing-your-employees-mental-health-at-work
Air Pollution and Atopic Dermatitis, from Molecular Mechanisms to Population-Level Evidence: A Review. (n.d.). PMC. Retrieved July 26, 2025, from https://pmc.ncbi.nlm.nih.gov/articles/PMC9916398/
Air Pollution Linked to Worse Atopic Dermatitis. (n.d.). American Journal of Managed Care. Retrieved July 26, 2025, from https://www.ajmc.com/view/air-pollution-linked-to-worse-atopic-dermatitis
Altius Group. (n.d.). Reduce Mental Health Stigma in the Workplace. Retrieved July 26, 2025, from https://altius.au/news-and-research/5-strategies-to-reduce-mental-health-stigma-in-your-workplace
Biologics – Northeast Allergy, Asthma, and Immunology. (n.d.). Northeast Allergy, Asthma, and Immunology. Retrieved July 26, 2025, from https://neaai.com/biologics/
Biologics for Asthma & Eczema. (n.d.). Maryland Allergy and Asthma Center. Retrieved July 26, 2025, from https://www.mdaac.com/biologics-for-asthma-eczema
Calm Health. (n.d.). A Leader’s Guide to Supporting Mental Health Conversations at Work. Retrieved July 26, 2025, from https://health.calm.com/resources/blog/talking-about-mental-health-at-work/
Fertifa. (n.d.). Why Mental Health Training for Managers Should Be Top of Your HR Agenda. Retrieved July 26, 2025, from https://www.fertifa.com/post/mental-health-training-for-managers
Genetic factors predisposing to atopic dermatitis: selected genes related to the skin barrier and immune response. (n.d.). Termedia. Retrieved July 26, 2025, from https://www.termedia.pl/Journal/-123/pdf-56099-10?filename=Genetic%20factors.pdf
Genetics and Epigenetics of Atopic Dermatitis: An Updated Systematic Review. (n.d.). MDPI. Retrieved July 26, 2025, from https://www.mdpi.com/2073-4425/11/4/442
Interactions Between Atopic Dermatitis and Staphylococcus aureus Infection: Clinical Implications. (n.d.). PMC. Retrieved July 26, 2025, from https://pmc.ncbi.nlm.nih.gov/articles/PMC6658404/
Mechanisms by Which Atopic Dermatitis Predisposes to Food Allergy and the Atopic March. (n.d.). PMC. Retrieved July 26, 2025, from https://pmc.ncbi.nlm.nih.gov/articles/PMC6267189/
Microbiome in the Gut-Skin Axis in Atopic Dermatitis. (n.d.). PMC. Retrieved July 26, 2025, from https://pmc.ncbi.nlm.nih.gov/articles/PMC6021588/
Microbiome of the Skin and Gut in Atopic Dermatitis (AD): Understanding the Pathophysiology and Finding Novel Management Strategies. (n.d.). PubMed Central. Retrieved July 26, 2025, from https://pmc.ncbi.nlm.nih.gov/articles/PMC6518061/
Mind. (n.d.). Mental health training. Retrieved July 26, 2025, from https://www.mind.org.uk/workplace/mind-training/
nilo. (n.d.). Workplace Mental Health Programs: A complete guide. Retrieved July 26, 2025, from https://nilohealth.com/blog/workplace-mental-health-programs/
Pathogenesis and management of atopic dermatitis: insights into epidermal barrier dysfunction and immune mechanisms. (n.d.). Open Exploration Publishing. Retrieved July 26, 2025, from https://www.explorationpub.com/Journals/eaa/Article/100973
PHW NHS Wales. (n.d.). Guidance for managers in supporting the disclosure of mental health problems by employees. Retrieved July 26, 2025, from https://phw.nhs.wales/about-us/policies-and-procedures/policies-and-procedures-documents/human-resources-policies-supporting-documents/guidance-for-managers-in-supporting-the-disclosure-of-mental-health-problems-by-employees-docx/
Rethink. (n.d.). Confidentiality and your mental health information. Retrieved July 26, 2025, from https://www.rethink.org/advice-and-information/rights-laws-and-criminal-justice/your-rights/confidentiality-and-your-mental-health-information/
Return To Work. (n.d.). Confidentiality and privacy – Return To Work. Retrieved July 26, 2025, from https://returntowork.workplace-mentalhealth.net.au/confidentiality-and-privacy/
SHRM. (2024). Toolkit: Creating a Mental-Health-Friendly Workplace. Retrieved July 26, 2025, from https://www.shrm.org/topics-tools/tools/toolkits/mental-health-friendly-workplace
Skin Exposure and Asthma: Is There a Connection?. (n.d.). PMC – PubMed Central. Retrieved July 26, 2025, from https://pmc.ncbi.nlm.nih.gov/articles/PMC3266020/
Spill. (n.d.). An employer’s duty of care and legal responsibilities for mental health at work. Retrieved July 26, 2025, from https://www.spill.chat/mental-health-at-work/an-employers-duty-of-care-and-legal-responsibilities-for-mental-health-at-work
Spring Health. (n.d.). Overcoming Mental Health Stigma: A Guide for HR Leaders. Retrieved July 26, 2025, from https://www.springhealth.com/blog/overcoming-mental-health-stigma-guide-hr-leaders
Staphylococcus aureus and its IgE-inducing enterotoxins in asthma: current knowledge. (n.d.). ERS Publications. Retrieved July 26, 2025, from https://publications.ersnet.org/content/erj/55/4/1901592
The Atopic March: Progression from Atopic Dermatitis to Allergic Rhinitis and Asthma. (n.d.). PMC. Retrieved July 26, 2025, from https://pmc.ncbi.nlm.nih.gov/articles/PMC4240310/
The Psychology of Atopic Dermatitis. (n.d.). MDPI. Retrieved July 26, 2025, from https://www.mdpi.com/2077-0383/13/6/1602
The Roles of Th2-Type Cytokines in the Pathogenesis of Atopic Dermatitis. (n.d.). SciSpace. Retrieved July 26, 2025, from https://scispace.com/pdf/the-roles-of-th2-type-cytokines-in-the-pathogenesis-of-1841wnkviy.pdf
ThoughtFull World. (n.d.). The Role of HR in Employee Mental Health. Retrieved July 26, 2025, from https://www.thoughtfull.world/resources/blog/the-role-of-hr-in-employee-mental-health
TriNet. (n.d.). Best Practices for Discussing Mental Health in the Workplace. Retrieved July 26, 2025, from https://www.trinet.com/insights/best-practices-for-discussing-mental-health-in-the-workplace
Type 2 immunity in the skin and lungs. (n.d.). PubMed. Retrieved July 26, 2025, from https://pubmed.ncbi.nlm.nih.gov/32319104/
UNLEASH. (n.d.). What is the role of HR in employee mental health: how to approach mental health challenges in the workforce?. Retrieved July 26, 2025, from https://www.unleash.ai/what-is-the-role-of-hr-in-employee-mental-health-how-to-approach-mental-health-challenges-in-the-workforce/
Understanding the psychological toll of asthma or allergies. (n.d.). News-Medical.net. Retrieved July 26, 2025, from https://www.news-medical.net/news/20250507/Understanding-the-psychological-toll-of-asthma-or-allergies.aspx
World Health Organization. (2019). Mental health in the workplace. Retrieved July 26, 2025, from https://www.who.int/news-room/fact-sheets/detail/mental-health-in-the-workplace



Leave Your Comment